Bioimage Gallery
Welcome to our image gallery, where you can see some of the best and latest images captured using our 3D real-time imaging technique. These live-cell images were taken using our 'bolt-on' optical system, designed for use with commercial microscopes.
Animal Cells:
- Chinese Hamster Ovary (CHO) cells
- Drosophila oocyte
- Fluorescence imaging
- DIC images of mitotic HeLa cells
- DIC images of vesicles within apoptotic HeLa cells - imaging on 9 planes!
- African green monkey kidney (Vero) cells
- Human sperm cells
- Human sperm cells 2 - imaging on 9 planes!
HeLa cancer cells:
Plant Cells:
Acknowledgements
Note: The abbreviation "Δz" means the separation in depth (z-axis) of each subsequent imaged plane. Therefore, when three planes are imaged simultaneously they are images taken over a total depth range of 2Δz.
Animal Cells:
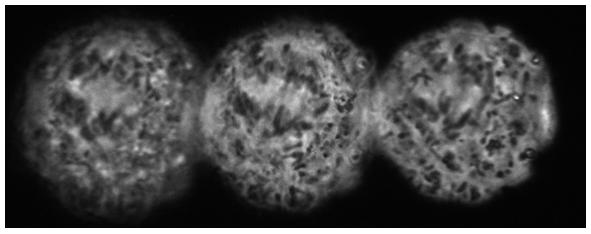
Chinese Hamster Ovary (CHO) cells
Collaboration with: Dr Lynn Paterson from the School of Engineering and Physical Sciences, Heriot-Watt University.
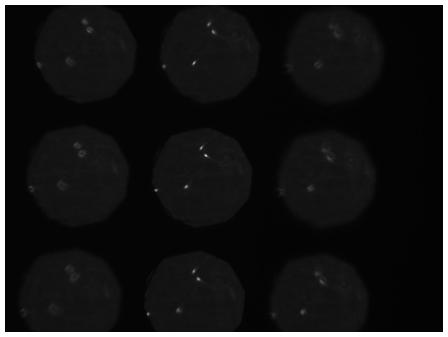
Sample: Chinese Hamster Ovaries (CHO) with DsRed present in cell mitochondria
Imaging mode: Bright field illumination with 600nm LED, fluorescence with 532nm laser through objective
Magnification: 100x (oil immersion)
Imaged plane separation: Δz = 1.1μm
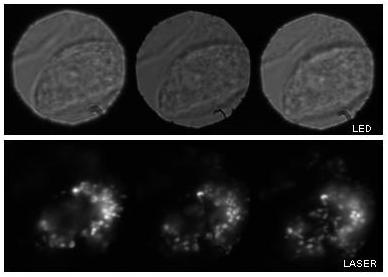
Bright field and fluorescence multi-plane imaging of CHO cells. The cells appear 'balled up' perhaps as a precursor to cell division.
back to top
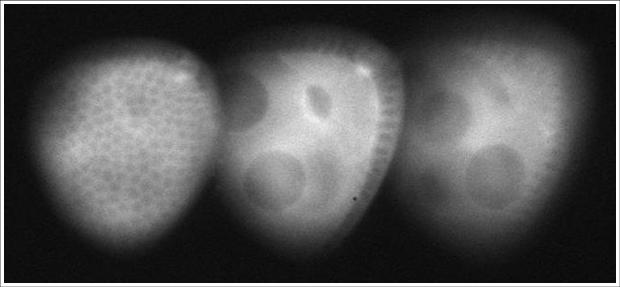
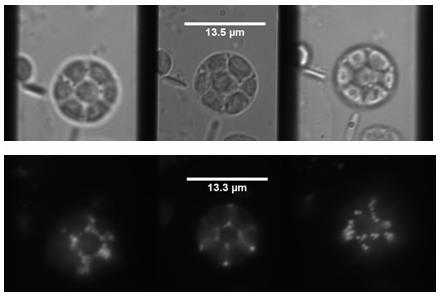
Drosophila oocyte
Collaboration with: Prof Ilan Davis, Dr Richard Parton and Dr Kirsten Lillie from Department of Biochemistry, Oxford University.
Sample: GFP present in Drosophila ovary cell
Imaging mode: Fluorescence GFP filter (470nm excitation, emission filtered by the narrowband 3D "bolt-on" optics to 520nm ±5nm)
Magnification: 100x (oil immersion)
Imaged plane separation: Δz = 7.3 μm between each image plane
Partial view of a stage 8 Drosophila egg chamber. Left-most image: the small dark spheres in focus are the follicle cells, in the central image the oocyte is in the focus. The large dark spheres are two nurse cell nuclei.
back to top
HeLa cancer cells
Fluorescence imaging
Collaboration with: Dr David Stephens from the Department of Biochemistry, University of Bristol
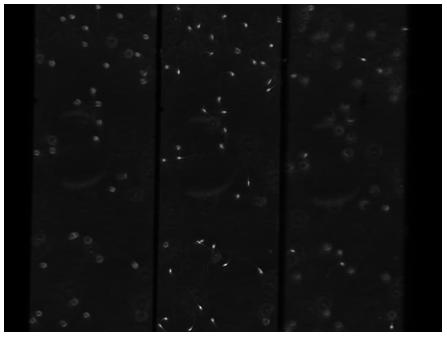
Sample: HeLa cancer cells with GFP labelled secretory cargo exit sites on the endoplasmic reticulum (labelled with GFP-Sec23)
Imaging mode: Fluorescence with 470 nm laser through objective
Magnification: 100x (oil immersion)
Imaged plane separation: Δz = 1.07μm
"HeLa" is a common cell line used in cancer research. The cell line was derived from cervical cells and is termed 'immortal' in that they can divide an unlimited number of times in a laboratory cell culture plate as long as fundamental cell survival conditions are met (i.e. being maintained and sustained in a suitable environment). Here we see 3D fluorescence images of a HeLa cell with GFP labelled secretory cargo exit sites on the endoplasmic reticulum (labelled with GFP-Sec23).
back to top
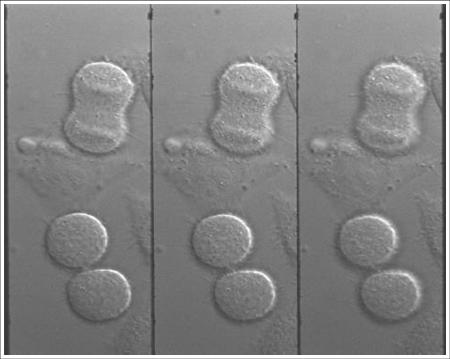
DIC images of mitotic HeLa cells
Collaboration with: Prof Viki Allan, Dr Peter March and Ms Rachel Southern, from the Faculty of Life Sciences, University of Manchester
Sample: Mitotic live HeLa (human cancer) cells
Imaging mode: DIC imaging with a 525nm LED (later filtered by the narrowband 3D "bolt-on" optics to 520nm ±5nm)
Magnification: 60x
Imaged plane separation: Δz = 1.63 μm between each image plane
Mitotic HeLa cells. The upper cell is in the process of dividing, the lower cell pair has just finished. In the upper cell chromosomes (horizontal structures) are shown and in the lower cells vesicles (small bright spots) can be seen.
back to top
DIC images of vesicles within apoptotic HeLa cells - 9 plane imaging
Collaboration with: Prof Viki Allan, Dr Peter March and Ms Rachel Southern, from the Faculty of Life Sciences, University of Manchester
Sample: Apoptotic HeLa (human cancer) cells
Imaging mode: DIC imaging with a 525nm LED (later filtered by the narrowband 3D "bolt-on" optics to 520nm ±5nm)
Magnification: 60x
Imaged plane separation: Δz = 0.81 μm between each image plane

Vesicles within an apoptotic HeLa cell imaged on nine planes with crossed gratings. Reduced field of view has excluded the plasma membrane.
back to top
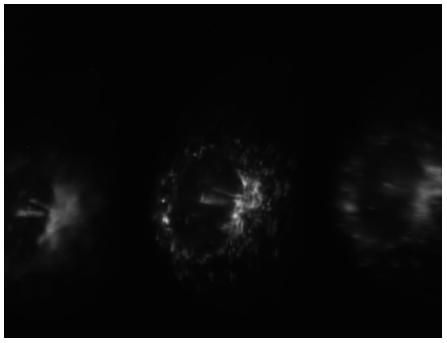
African green monkey kidney (Vero) cells
Collaboration with: Prof Viki Allan and Dr Peter March from the Faculty of Life Sciences, University of Manchester
Sample: African green monkey kidney (vero cells) undergoing cell division
Imaging mode: Phase contrast imaging in white light (later filtered by the narrowband 3D "bolt-on" optics to 520nm ±5nm)
Magnification: 60x
Imaged plane separation: Δz = 1.5μm
3D Phase Contrast Imaging of African Green Monkey Kidney cells during mitosis (anaphase). Chromatid pairs begin to separate to form two daughter chromasomes. These are then pulled apart, to opposite sides of the cell, so that when it divides one copy of each chromasome will be present in the two daughter cells. In these images we see the chromatid pairs aligning onto the spindle (clear stripe, upper left hand region of each image) prior to being pulled apart.
back to top
Human sperm cells
Collaboration with: Dr Jackson Kirkman-Brown, and Dr Dave Smith of the Centre for Human Reproductive Science (ChRS), Assisted Conception Unit, Birmingham Women's Health Care NHS Trust. Jeremy Graham (Fellow of the ChRS) of Cairn Research Ltd
Sample: Human live sperm cells in viscous media
Imaging mode: Phase contrast imaging in white light (later filtered by the narrowband 3D "bolt-on" optics to 520nm ±5nm)
Magnification: 20x
Imaged plane separation: Δz = 14.6μm
3D Phase-contrast image of motile human sperm on 3 image planes separated by 14.6μm. This figure shows a single frame from a time-lapse sequence recorded at 50Hz.
back to top
Human sperm cells 2 - 9 plane imaging
Collaboration with: Dr Jackson Kirkman-Brown, and Dr Dave Smith of the Centre for Human Reproductive Science (ChRS), Assisted Conception Unit, Birmingham Women's Health Care NHS Trust. Jeremy Graham (Fellow of the ChRS) of Cairn Research Ltd
Sample: Human live sperm cells in viscous media
Imaging mode: Phase contrast imaging in white light (later filtered by the narrowband 3D "bolt-on" optics to 520nm ±5nm)
Magnification: 20x
Imaged plane separation: Nine planes imaged by crossing two quadratic gratings Δz = 3.66μm (100λ wave grating) and Δz = 910nm (25λ grating)
Notes: This data was taken as a quick preliminary experiment to investigate the possibility of imaging on nine planes. Therefore the gratings used were not optimized for this purpose - hence the non-uniform z-plane separations, the loss of part of the 3rd row of images and the slightly-squint arrangement of the 9 images
3D Phase-contrast image of motile human sperm on 9 image planes separated by 3.56μm (horizontal axis) and 910nm (vertical axis). The 9 simultaneous z-planes are obtained by crossing 2 curved gratings. The figure shows a single frame from a time-lapse sequence recorded at 50Hz.
back to top
Plant Cells:
Arabidopsis, mutant plant cells
Collaboration with: Dr David Logan School of Biology, St Andrews University (at time of capture, currently at University of Saskatchewan, Canada)
Sample: 14 day-old Arabidopsis seedling expressing GFP targeted to mitochondria
Imaging mode: Bright field illumination with 520nm LED, fluorescence with 470nm laser through objective
Magnification: 100x (oil immersion)
Imaged plane separation: Δz = 1μm
3D Bright Field and Fluorescence images of mutant Arabidopsis cells are shown. Notice there are no cell walls in this sample - this is due to mutation.
back to top
Acknowledgements
We wish to thank all of the collaborators named here, who have offered to us invaluable advice about the biological applications and processes involved in the cells imaged.
In addition we wish to acknowledge funding from the Engineering and Physical Sciences Research Council (Grant numbers GR/R32185, GR/S96555 and EP/E01500X) and the Science and Technology Facilities Council (Grant numbers PP/E005047, ST/F001754 and ST/F003463).
back to top
Our Research
Find information on our current research projects, and our research interests both past and present...
Life Sciences Interface
- Introduction
- Real-time 3D imaging
- 3D Live-cell imaging
- Particle Tracking
- Sperm Motility
- Bioimage Gallery